sodium sulfate molar mass|what is na2so4 in chemistry : Baguio Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. . See more Resultado da 9 de ago. de 2023 · Não existem reclamações sobre trioderm plus porque ele foi criado após exaustivos estudos e escolhas minuciosas de seus .
0 · what is na2so4 in chemistry
1 · sodium sulfate hazards
2 · sodium sulfate formula mass
3 · sodium sulfate common name
4 · sodium sulfate 3d model jsmol
5 · how many grams in na2so4
6 · boiling point of sodium sulfate
7 · anhydrous sodium sulfate boiling point
WEBUnilabs Santa Maria da Feira | Edifício D.Manuel. Rua Dr. Alcides Strecht Monteiro, 55 (Edifício D. Manuel), 4520-179 Santa Maria Da Feira. Temporariamente fechado. .
sodium sulfate molar mass*******Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. . See more• Anhydrous sodium sulfate, known as the rare mineral thenardite, used as a drying agent in organic synthesis.• Heptahydrate sodium sulfate, a very rare form.• Decahydrate sodium sulfate, known as the mineral See moreThe decahydrate of sodium sulfate is known as Glauber's salt after the Dutch–German chemist and apothecary Johann Rudolf Glauber (1604–1670), . See more
Sodium sulfate has unusual solubility characteristics in water. Its solubility in water rises more than tenfold between 0 °C and 32.384 °C, . See moreThe world production of sodium sulfate, almost exclusively in the form of the decahydrate, amounts to approximately 5.5 to 6 million tonnes annually (Mt/a). In 1985, production was . See more
Sodium sulfate is a typical electrostatically bonded ionic sulfate. The existence of free sulfate ions in solution is indicated by the easy formation of insoluble sulfates when these solutions are treated with Ba or Pb salts:Na2SO4 + BaCl2 . See moreCrystals of the decahydrate consist of [Na(OH2)6] ions with octahedral molecular geometry. These octahedra share edges such that 8 of the . See moreCommodity industriesWith US pricing at $30 per tonne in 1970, up to $90 per tonne for salt cake quality, and $130 for better grades, sodium sulphate is a very . See moreThere are 4 easy steps to find the molar mass of Na2SO4 based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Sodium .Na2SO4 (Sodium sulfate) molar mass. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of Na2SO4 (Sodium sulfate) is 142.0421 g/mol. .
Sodium sulfate. Formula: Na 2 O 4 S. Molecular weight: 142.042. IUPAC Standard InChI: InChI=1S/2Na.H2O4S/c;;1-5 (2,3)4/h;; (H2,1,2,3,4)/q2*+1;/p-2. Copy Sheet of paper on top of another sheet. .
Sodium Sulfate molecular weight. Molar mass of Na2SO4 = 142.04214 g/mol. Convert grams Sodium Sulfate to moles. or. moles Sodium Sulfate to grams. Molecular weight .
sodium sulfate molar massThe molar mass of Na2SO4 (Sodium sulfate) is: 142.034 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the .
There are 4 easy steps to finding the molar mass of Na2*SO4 based on its chemical formula.Formula: Na 2 O 4 S. Molecular weight: 142.042. IUPAC Standard InChI:InChI=1S/2Na.H2O4S/c;;1-5 (2,3)4/h;; (H2,1,2,3,4)/q2*+1;/p-2 Copy. IUPAC . The molar mass of a substance is the mass in grams of 1 mole of the substance. As shown in this video, we can obtain a substance's molar mass by summing the molar masses of its component atoms. We can then use the calculated molar mass . Our molar mass calculator comes to the rescue if you need to quickly check the weight of 1 mole of any element or chemical compound and you are unable to . Explanation of how to find the molar mass of Na2SO4: Sodium sulfate.A few things to consider when finding the molar mass for Na2SO4:- make sure you have the .The first step to finding the molar mass of Sodium Sulfate is to count the number of each atom present in a single molecule using the chemical formula, (SO4)Na2: Element Number of Atoms; S (Sulphur/Sulfur) 1: O (Oxygen) 4: Na .
Finally, add together the total mass of each element to get the molar mass of SO4Na2: 32.065 g/mol + 63.9976 g/mol + 45.97953856 g/mol = 142.04213856 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in SO4Na2, divide each total from step 3 by the total molar mass found in step 4:
David Park. 4 years ago. First, you can calculate the molar mass of FeCl2 by adding the molar masses of Fe (55.845 g/mol) and 2 atoms of Cl (2 times (35.446 g/mol). This gives a .Sodium sulfate. Molecular Formula NaOS. Average mass 142.042 Da. Monoisotopic mass 141.931274 Da. ChemSpider ID 22844. - Charge.7727-73-3. Glauber's salt. Disodium sulfate decahydrate. Sulfuric acid disodium salt, decahydrate. View More. Molecular Weight. 322.20 g/mol. Computed by PubChem 2.2 (PubChem release 2021.10.14) Component Compounds.what is na2so4 in chemistry The molar mass sodium sulfate, molar mass is 142.04 grams per mole. So given everything I've now told you, see if you can pause this video and figure out the molarity of this .
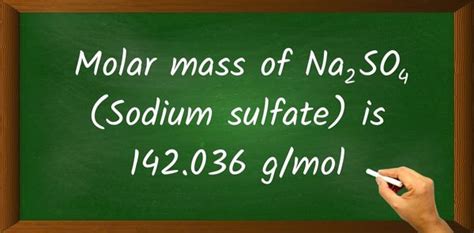
Sodium Sulfate Na2SO4 Molecular Weight, molar mass converter. ENDMEMO . Na 2 SO 4 Molar Mass Converter. Weight: Mole: Na 2 SO 4 Molar Mass Conversion in Batch. Weight: Mole: Note: Fill in one box to get results in the other box by clicking "Calculate" button. Data may be separated by semicolon (;), space, tab, or in separated lines. . Find the molar masses of carbon (C), hydrogen (H), and oxygen (O). Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol.
The molar mass will be equal to: (1 atom x 56 grams/mole Fe) + (2 atoms x 35.5 grams/mole of chlorine) = 127 grams/mole of iron (II) chloride. For other compounds, this might get a little bit more complicated. For example, take the example of zinc nitrate, or Zn (NO 3) 2. In this compound, we have one atom of zinc, two atoms of nitrogen (one .
sodium sulfate molar mass what is na2so4 in chemistrySO 4. 142.0421 g/mol. The molar mass and molecular weight of Na 2
Notes. Go To: Top Data from NIST Standard Reference Database 69: NIST Chemistry Book The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.Na2SO4 molecular weight. Molar mass of Na2SO4 = 142.04214 g/mol. This compound is also known as Sodium Sulfate. Convert grams Na2SO4 to moles. or. moles Na2SO4 to grams. Molecular weight calculation: 22.98977*2 + 32.065 + 15.9994*4.Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Na2SO4*10H2O: Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. S (Sulphur/Sulfur) 1 × 32.065 = 32.065. O (Oxygen)SO 4. 142.0421 g/mol. The molar mass and molecular weight of Na 2
Notes. Go To: Top Data from NIST Standard Reference Database 69: NIST Chemistry Book The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.Na2SO4 molecular weight. Molar mass of Na2SO4 = 142.04214 g/mol. This compound is also known as Sodium Sulfate. Convert grams Na2SO4 to moles. or. moles Na2SO4 to grams. Molecular weight calculation: 22.98977*2 + 32.065 + 15.9994*4.Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Na2SO4*10H2O: Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. S (Sulphur/Sulfur) 1 × 32.065 = 32.065. O (Oxygen)Sodium sulfate. Formula: Na 2 O 4 S; Molecular weight: 142.042; . Notice: Concentration information is not available for this spectrum and, therefore, molar absorptivity values cannot be derived. Additional Data. View scan of original (hardcopy) spectrum. View image of digitized spectrum (can be printed in landscape orientation).
45.0554. Computing molar mass step by step. First, compute the number of each atom in Na 2 SO 4: Na: 2, S: 1, O: 4. Then, lookup atomic weights for each element in periodic table: Na: 22.98976928, S: 32.065, O: 15.9994. Now, compute the sum of products of number of atoms to the atomic weight: Molar mass (Na 2 SO 4) = ∑ Count i * Weight i =. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass .
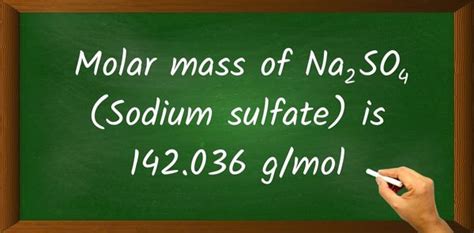
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound. Printed from https://www.webqc.org. Molar Mass, Molecular Weight and Elemental Composition Calculator . Sodium hydrogen sulfate: Formula in Hill system is H 14 Na 2 O 11 S: Computing molar mass (molar weight)3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in MgSO4: Molar Mass (g/mol) Mg (Magnesium) 1 × 24.305 = 24.305. S (Sulphur/Sulfur) 1 .
Molar mass: Variable; typically around 421 g/mol . Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), also called sodium alkylethersulfate, is an anionic detergent and surfactant found in many personal care products (soaps, shampoos, toothpaste, etc.) and for industrial uses.The first step to finding the molar mass of Sodium Sulfate Decahydrate is to count the number of each atom present in a single molecule using the chemical formula, Na2(SO4)(H2O)10: Element Number of Atoms; Na (Sodium) 2: S (Sulphur/Sulfur) 1: O (Oxygen) 14: H (Hydrogen) 20: 2. Find Atomic Mass of Each ElementThe molar mass for this compound is computed to be 176.124 g/mol. The given number of moles is a very small fraction of a mole (~10 −4 or one-ten thousandth); therefore, we would expect the corresponding mass to be about one-ten thousandth of the molar mass (~0.02 g). Performing the calculation, we get:
Resultado da O AnonyTun é uma ferramenta completa VPN para Android que te permite navegar pela Internet no modo anônimo e acessar sites bloqueados no .
sodium sulfate molar mass|what is na2so4 in chemistry